This is a word cloud
of terms in the abstracts of all articles published by our group during 1990 –
2014; it captures the gist of our research interests during that time.
My major more current
research interests are evolutionary genetics, in particular the
wide-ranging ramifications of genetic conflict, and evolutionary ecology, broadly defined to include the interactions
between behaviour, morphology, physiology and
genetics on one hand and the environment on the other. Several current research
projects in my laboratory focus, in one way or another, on understanding the
causes and consequences of interactions between the sexes and the genetic /
evolutionary consequences of having two sexes. We currently follow a few main
themes:
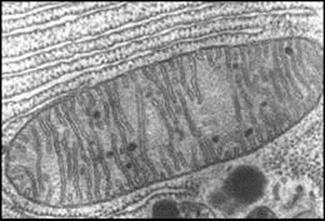
- Every animal cell contains two types of
genetic material: genes in the nucleus and genes in the mitochondria. The
mitochondrial genome is small but encodes for products that are vital for the
metabolic performance of the cell: the energy producing enzymatic machinery of
the mitochondria is built collectively by products of the mitochondrial and
nuclear genome. MtDNA has long been considered selectively neutral. However,
recent research in our laboratory and many others have demonstrated that mtDNA
variation across and within populations interacts with nuclear genes in
affecting important phenotypic traits such as metabolic phenotypes, development
rate and even fitness. Our research ultimately aims to understand the role of
mtDNA in evolutionary adaptation. Moreover, mtDNA does not follow the same
evolutionary “rules” as does nDNA, chiefly because it
is haploid, does not recombine and is maternally inherited. There is therefore
generally no selection on mtDNA in males and we are currently exploring the
possibility that sexual conflict over optimal life histories have important
consequences for mitonuclear coevolution.
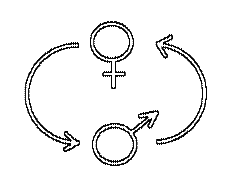
- Some of our
research revolves, directly or indirectly, around the causes and consequences
of conflicts of interest between the sexes. Such conflicts can arise because
the direction of selection on an allele at a given locus depends upon in which
sex it is expressed, such that one allele yields highest fitness when expressed
in males and another when expressed in females. This can even lead to the
adaptive evolution of lowered population fitness! Alternatively, sexual
conflict can generate "arms races" between the sexes, known as
sexually antagonistic coevolution. Our empirical work is done primarily with a
variety of different insects. In collaboration with researchers in USA, Canada,
Australia and England, we aim both at characterizing various male-female
conflicts, and at understanding the far-reaching consequences and
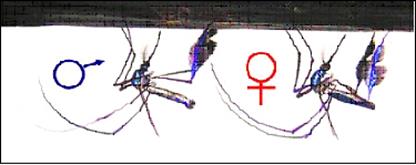
coevolutionary dynamics of these types of genetic conflicts. Below are pictures
of three of our model systems: a Sabethine mosquito
where both sexes carry striking leg ornaments, a male and a female water
strider engaged in a premating “struggle” and a mating pair of seed beetles (Callosobruchus maculatus).


-
More than a century after Darwin, our understanding of the process by which new
species are formed is still incomplete. In our lab, we are aiming at
illuminating the processes involved both in the evolution of reproductive
isolation (speciation) and in evolution leading to extinction of lineages. We
are interested in understanding (1) the evolution of hybrid inviability, (2)
the evolution of "cryptic" reproductive isolation that occurs after
mating, such that females may mate with males of both their own and other
species but yet will produce no or little hybrid offspring, (3) if and how
sexual selection contributes to the evolution of species specific “signals”,
including male seminal fluid proteins transferred to females at mating and (4)
which gene families diverge most rapidly upon speciation. The last point is
addressed using comparative genomics, within and between species, of seed
beetles.
BACK
to the main page
